Lisfranc
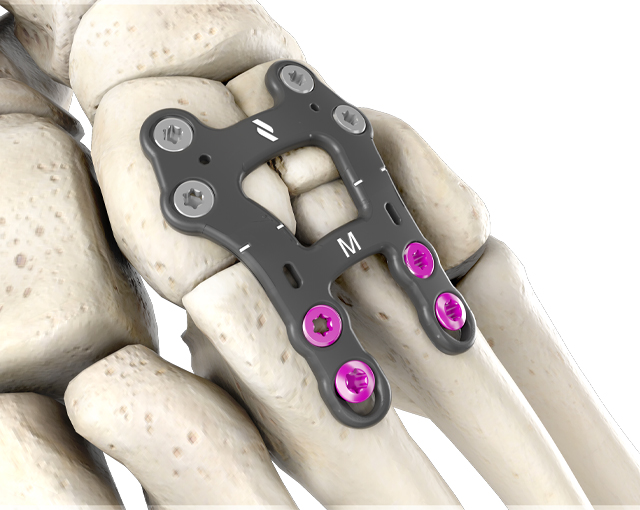
The 1.5mm profile of the lisfranc plates provides strength and stability as it addresses patients with shallow tissue envelopes. These plates are precontoured with a built-in ridge angle to accommodate metatarsal declination.
This anatomic bend reduces the risk of accidental ray elevation during fixation, which can lead to transfer lesions of the adjacent metatarsals. All plates allow for the option of locking and non-locking Ø3.0mm or Ø3.5mm screws and include an oblong hole for additional metatarsal compression and fixation.

Lisfranc
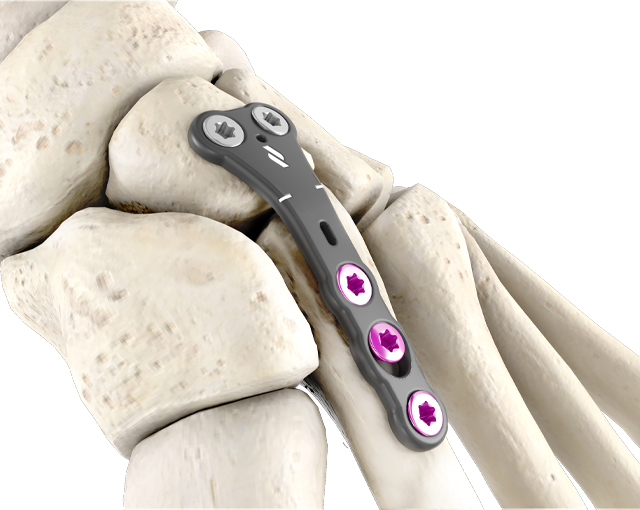
The 1.5mm profile of the lisfranc plates provides strength and stability as it addresses patients with shallow tissue envelopes. These plates are precontoured with a built-in ridge angle to accommodate metatarsal declination.
This anatomic bend reduces the risk of accidental ray elevation during fixation, which can lead to transfer lesions of the adjacent metatarsals. All plates allow for the option of locking and non-locking Ø3.0mm or Ø3.5mm screws and include an oblong hole for additional metatarsal compression and fixation.

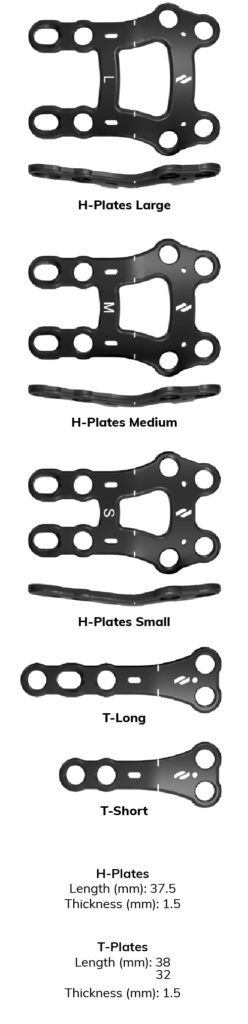

The Airlock® Ø3.0mm and Ø3.5mm locking and non-locking screws may be used in all Airlock plate fixation holes; however, the compression holes only accommodate Ø3.0mm non-locking screws. A Nexis Ø4.0mm headless compression screw of appropriate length may be used for additional plantar compression. All instrumentation is conveniently organized and color coded (see below).

Monoaxial & Polyaxial Capability
- Monoaxial locking screws
- Polyaxial non-locking screws
- Tapered head
- Self-tapping design
- Self-retaining driver / screw interface
Airlock with Presslock Upgrade

Airlock 2.0

Airlock 2.0 Reduction Instruments

AirlockGO® Product Delivery Option
AirlockGoTM combines Novastep’s Airlock® plating system and cleanSTART® sterile packaged, single-use instrument kits with a selection of Nexis® Ø4.0mm headless compression screws; providing the user with a self-contained, all-inclusive unit that addresses a range of foot and ankle pathologies.
Each AirlockGOTM box features a unique, procedurespecific configuration of implants and instruments that includes precontoured low-profile plates, monoaxial locking, polyaxial non-locking and headless compression screws.
All instrument kits and implants are individually packaged sterile with self-adhesive, UDI compliant data matrix labels.